Mylan Responds with a Press Release that Dodges Concerns and Questions
On January 20th, 2017, almost exactly eleven years to the day since I was thrown into this work in the food allergy world, I heard from from Mylan’s communication’s department for the first time. They wanted to discuss points made in a recent FOX interview. I was grateful. After a decade of working in this space, they finally wanted to talk.
So I turned to our community for feedback, prior to the call.
But the call didn’t happen. Instead, the communications team responded with a press release.
When I first saw it, I sent an email suggesting that we get on a call, as the press release fails to address so many of the concerns that our community has.
But I haven’t heard back.
Since Mylan continues to follow what is written here, we have decided to post the concerns from our community, along with their press release. Thank you so much to all of you who have written, responded and called.
- Mylan refers to EpiPen users as “patients.” Referring to our children and loved ones as “patients” suggests that we are sick. While that may be how the company recognizes EpiPens recurring revenue model, many food allergy consumers are healthy, read labels, and many avoid additives, artificial ingredients, GMOs and other synthetic ingredients. Countless times, the community have said that food allergies are a blessing in disguise, as it taught them how to make smarter and healthier food choices. Perhaps rather than using the word “patient” as they do below, Mylan can stick with consumer or even “user” (a term used by Coca Cola).
- Our community is composed of pediatricians, allergists, TV producers, journalists, attorneys, MDs, parents, teachers, nurses, investment bankers, Congressmen and women and so many more. Again, “patient” minimizes the incredible diversity of the food allergy world.
- The press release below fails to answer a question asked by many allergists: how did Mylan secure exclusivity with the pharmacy benefit managers? Was a deal cut? Did money change hands to secure that EpiPen was the only device offered? Under what terms was Mylan able to secure the exclusivity? Was something done to ensure market foreclosure? Contracts between Mylan and the PBMs could answer that.
- Mylan suggests that they “invested over $1 billion in raising awareness and increasing education about the risks of anaphylaxis.”“Mylan increased its branded ad spending on the EpiPen by 357% over five years. During the same time the drugmaker hiked the price of the allergy auto-injector by 179%.Mylan spent $43 million on branded ad spending for the EpiPen in 2015, down slightly from $48.9 million in 2014 and up significantly from $9.4 million in 2011, according to data from Kantar Media. At the same time, the drugmaker has steadily increased the wholesale acquisition price, which excludes rebates and discounts, twice a year since 2011, according to Elsevier Clinical Solutions’ Gold Standard Drug Database. In May 2011 the EpiPen was priced at $164.98, and that price rose to $461 by May 2015. It now costs $608.61.”
- Given that the United States and New Zealand are the only two countries in the world that allow pharmaceutical advertising directed at the consumer, is the skyrocketing advertising budget the reason that Mylan jacked up the price of the EpiPen twice a year since 2011?
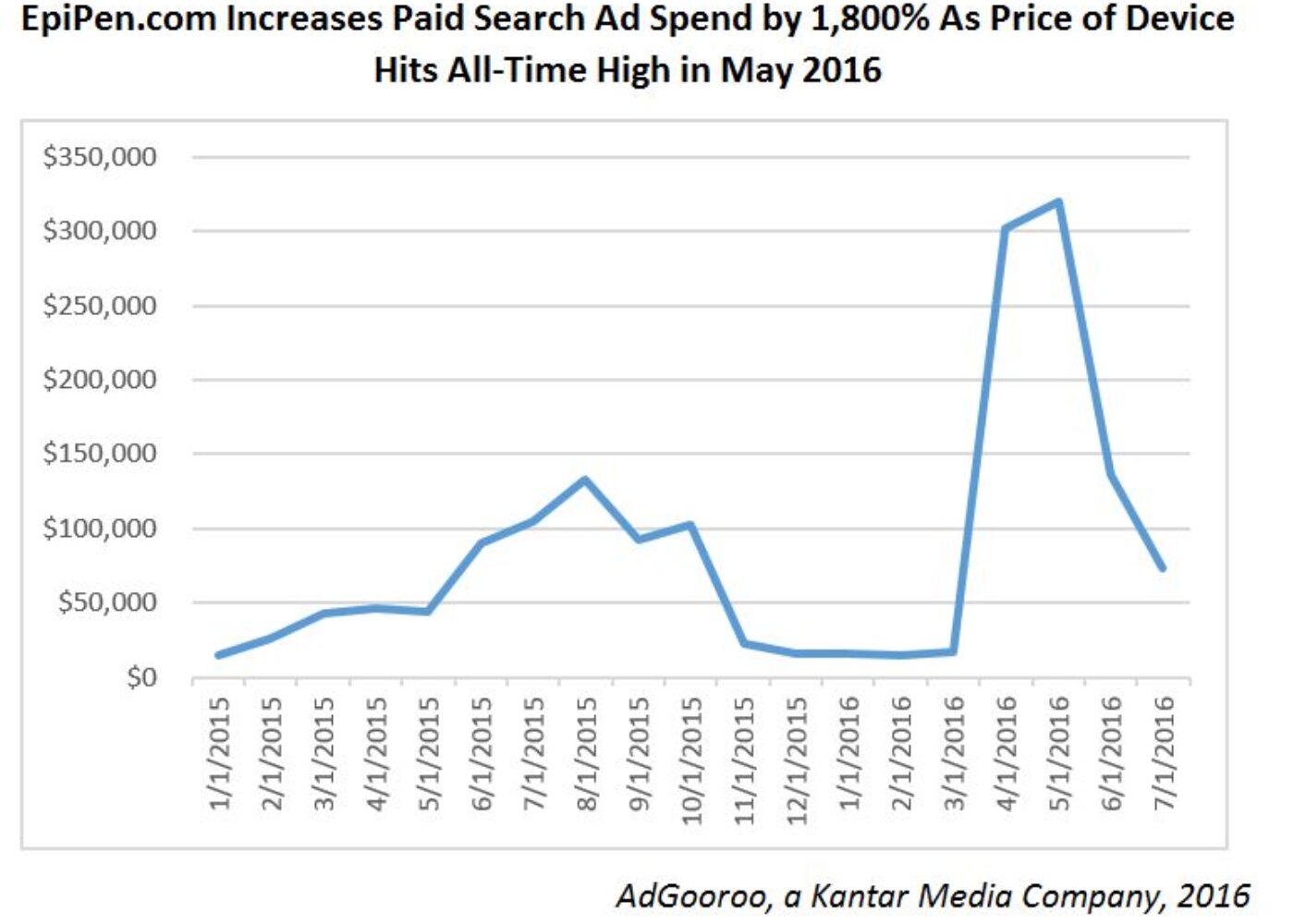
- How did the search ad spending drive pricing decisions? Mylan didn’t just shell out for TV ads as EpiPen prices soared. Search spending was way up, too. According to FiercePharma, “the company shelled out and worked hard to build EpiPen’s profile in the marketplace. Coupled with the company’s repeated price increases, its EpiPen marketing program built the epinephrine injector into a blockbuster product accounting for 20% of the company’s revenue. The thinking goes like this: Potential customers see an ad on TV, go to their smartphones and type a word or brand into Google search to find out more. Why not place an ad linked to allergy keywords?” EpiPen.com increased paid search ad spend by 1,800% as the price of the device hits an all time high in May 2016.
- In the fall of 2016, Mylan’s CEO was questioned by Congress in a Congressional hearing about Mylan’s market share. She acknowledged what has also been acknowledged that EpiPen had a 95% market share (Athenahealth states that EpiPen’s market share was 95.7% in August 2016). How was Mylan able to secure that monopoly? Was that exclusivity ensured through the product donations to schools?
- The release below does nothing to address consumer concerns that Mylan misrepresented its drug classification to Medicare and Medicaid. The drugmaker reached a $465 million settlement with the U.S. Justice Department and other government agencies to resolve questions over rebates required by the Medicaid program.
- And lastly, consumers have been asking for refunds since the price drop. As an anti-trust probe is launched, many are lining up for more answers.
Below is Mylan’s press release:
We appreciate the work that you do for the community and your role in continuing this important conversation. We reached out to you to address some of the concerns that have been raised. We value the comments we read from the community on your social media outlets, and given their engagement and interest in our discussion, we feel it is most appropriate to respond where the broader group can read it. While this post is lengthy, we wanted to address the many topics that were raised.
First and foremost, we were saddened to read about the boy who passed away on Thanksgiving after experiencing anaphylaxis, and we share our condolences with this family. While the collective severe allergy community has made progress over the years in raising awareness and increasing education, tragedies such as this demonstrate there’s more work to be done.
This is the primary reason why we have invested over $1 billion in raising awareness and increasing education about the risks of anaphylaxis. We worked to change laws to allow access to epinephrine in schools so it is readily available for any child who has an anaphylactic event. We have given away nearly 800,000 EpiPen Auto-Injectors to schools across America. These free devices have been used hundreds of times. We don’t want to go back to a time – not that long ago – when awareness of anaphylaxis was much lower and epinephrine auto injectors were only available in schools with a prescription for an individual child. Achieving this level of expansion of awareness requires a significant commitment.
Mylan takes its role within this community seriously and has been – and continues to be – committed to increasing awareness, preparedness and access to treatment.
- In regards to price, patients are rightfully concerned about rising drug prices. Mylan has felt the full weight of these concerns in relation to the price of EpiPen® (epinephrine injection, USP) Auto -Injector. However, it is important to understand that the way consumers pay for their healthcare has changed dramatically over the past few years. The stark reality is that a sharp rise in high deductible health plans has exposed more consumers to higher out-of-pocket costs for their medications. And working families will continue to face sticker shock at the pharmacy counter for medications and be forced to make difficult choices until the process of pricing pharmaceuticals is reformed to recognize the increasing shift of costs directly to consumers.
- As it relates to EpiPen Auto-Injector, last summer approximately 85% of consumers prescribed EpiPen had an out-of-pocket cost of less than $100 and a majority paid less than $50. However, as a result of the rise in high deductible plans, about 15% of EpiPen purchasers were exposed to a wholesale acquisition cost (WAC), or list price, of $600 or more. While some competitors have recently suggested that patients do not pay the WAC price, you and many in this community know firsthand that this is not always the case in today’s environment. This is why we took immediate and decisive action to cut the price of EpiPen Auto-Injector in half through the launch of our authorized generic and to adjust our enhanced patient programs and resources to help increase access for this patient population. This was the most direct way to reduce the cost for patients. Media coverage has noted how the recent Auvi-Q® announcement reflects the challenges facing our system Mylan offers the lowest WAC* price ($300 for the authorized generic for EpiPen Auto-Injector and $608 for EpiPen Auto-Injector) and cash cost/direct ship cost ($300) to all patients, not just those visiting a select retailer.
- There was some confusion from your readers about our resources; let us clarify.
- For all patients, the authorized generic for EpiPen Auto-Injector is now widely available at pharmacies across the U.S. at half the wholesale acquisition cost ($300) of EpiPen Auto-Injector.
- A savings card and relevant terms and conditions are available online
- If you are being charged a cost that significantly exceeds $300 for either dose of product, this is an error. Please contact Mylan Customer Relations at 1-800-395-3376 or [email protected].
- Mylan also provides a direct ship option that offers the authorized generic for EpiPen Auto-Injector at a cash price of $300, the lowest direct to patient cost.
- For commercially insured patients purchasing EpiPen Auto-Injector, we expanded our My EpiPen Savings Card® program.
- This savings card and relevant terms and conditions are available online
- For uninsured and underinsured patients, we doubled the eligibility for our patient assistance program to support those earning up to 400% of the Federal Poverty Level. This means an uninsured or underinsured family of four making up to $97,200 pays $0.
Information about all of these resources can be found on Mylan.com/EpiPenAccess.- Many have questioned why we did not just lower the cost of the branded product. It would not have ensured that EpiPen Auto-Injector would be made more affordable for patients, especially for the uninsured, underinsured and those with high deductible health plans. Because we cannot control the costs charged by additional parties involved in the pharmaceutical supply chain and reimbursement system in which we operate, and given contractual arrangements with customers, cutting the list price of the branded product woul d not have guaranteed that patients would pay a lower price at the pharmacy. Therefore, we introduced the authorized generic for EpiPen Auto-Injector, which is identical to the branded product, at 50% of the WAC price, and expanded our savings card and assistance programs to reach more patients.
- Contrary to what appears to be a popular misperception, patients at risk for anaphylaxis, a life-threatening allergic reaction, have – and always have had – various alternatives when it comes to epinephrine. For more than 10 years, the market has been a competitive one, providing consumers and healthcare professionals with numerous options for their prescribed epinephrine auto-injector.
- Recent media attention about the various epinephrine auto-injectors on the market has generated confusion about patient options. There are important differences between products in terms of the functionality and administration process. We suggest that all patients work with a healthcare professional to determine the option that’s best for them. All patients should receive training on proper administration of a prescribed epinephrine auto-injector in a physician’s office and practice between doctor office visits.
Mylan remains dedicated to meeting the needs of those at risk for anaphylaxis, and we are committed to providing access to EpiPen Au to-Injector. We understand the frustration of anyone facing barriers to access. We respect the opinions that have been shared and welcome continued feedback and dialogue. Our hope is that the conversation surrounding EpiPen Auto-Injector serves as a catalyst for reform and real transparency in pharmaceutical pricing.*The final retail cost to the patient for EpiPen Auto-Injector and the authorized generic will be dependent upon their insurance and any mark-ups within the supply chain.





